Abstract
Bacterial sepsis triggers profound dysfunction of the coagulation and fibrinolytic systems leading to disseminated intravascular coagulation (DIC), characterized by consumptive coagulopathy and bleeding. DIC may contribute to multiple organ failures due to occlusion of microvasculature and tissue hypoperfusion. Bacteria and/or bacteria-derived molecules initiate the activation of both the intrinsic/contact phase and extrinsic pathways of coagulation that activate factor X (FX). Active FX (FXa) is the enzyme of the prothrombinase complex that leads to thrombin generation and subsequent formation of fibrin clots. Arixtra® (Fondaparinux Sodium), a synthetic pentasaccharide, binds to antithrombin and thereby selectively inhibits FXa. Here, a baboon model of E. coli sepsis was used to investigate the effect of FXa inhibition on sepsis-induced activation of coagulation and fibrinolytic pathways.
Two experimental baboon groups were studied:(i) E. coli challenge (n=4) , (ii) E. coli + Arixtra®(n=4). E. coli (1-2 x 1010 CFU/kg, LD100 dose) was infused intravenously over a 2 hour period. Arixtra® (0.08mg/kg) was administered subcutaneously to achive therapeutic concentration at the time of E. coli challenge (T0). The activation of coagulation was studied by measuring plasma levels of inhibitory complexes of antithrombin (AT) with activated factors: FXa-AT, FXIa-AT, thrombin-AT (TAT) and FVIIa-AT.
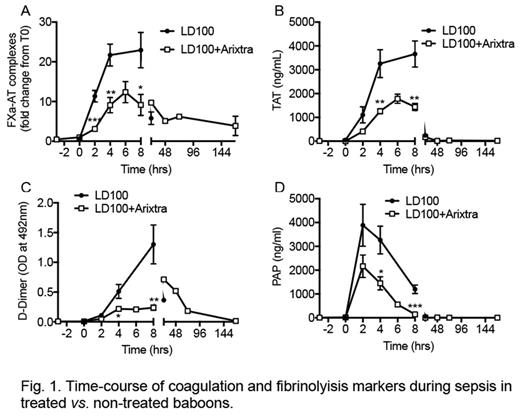
E. coli challenge induced the activation of coagulation pathway as early as 2 hours post-challenge as evident by increase in plasma level of FXa-AT and TAT complexes. Contact pathway activation (FXIa-AT), as well as FVII activation (FVIIa-AT), were significantly reduced in Arixtra® treated animals. FXa-AT and TAT complexes were maximally present at 4-8 hours post-challenge and were reduced by more than 50% in Arixtra® treated animals (Fig. 1, A and B). Similarly, Arixtra® reduced activated partial thromboplastin time and prothrombin time as well as fibrinogen consumption following E. coli challenge. In line with the above observation, the plasma levels of fibrinolysis markers, fibrinogen/fibrin degradation products, D-Dimer and plasmin-antiplasmin complex (PAP) were significantly reduced in the Arixtra® treated group (Fig. 1, C and D). Platelets consumption/thrombocytopenia was similar in both the Arixtra® treated and the untreated groups. Moreover, neutrophil activation as measured by myeloperoxidase activity in plasma was reduced in the Arixtra® treated group. Two out of four animals survived in Arixtra® treated groups compared to 100% mortality in untreated group [Log-rank (Mantel-Cox) test P=0.0037]. Notably, Arixtra® treated non-survivor animals demonstrated prolonged survival times.
Overall, the data suggests that Arixtra® reduced both coagulation and fibrinolytic pathway activation, thereby preventing DIC and providing survival benefits in E. coli sepsis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.